Laboratory Studies of HO2 Chemistry
ACD scientists have been studying the reaction kinetics and mechanisms of HO2 radicals using a number of complementary techniques.
HO2 radicals are a member of the HOx family, which is comprised of OH and HO2. Hydroxyl radicals, OH are responsible for the oxidation of many organic and inorganic pollutants which are emitted into the troposphere. Following a series of reactions, HO2 is usually produced, and then cycled back to OH by its reaction with nitric oxide, NO.
In the lower stratosphere, OH and HO2 radicals play a large role in controlling the concentration of ozone.
OH + O3 → HO2 + O2
HO2 + O3 → OH + 2O2
One way to terminate these oxidation chains is the self reaction of HO2 radicals.
HO2 + HO2 → H2O2 + O2
The rate coefficient for this particular reaction depends on both the temperature and pressure, and water vapor concentration. Scientists in ACD have measured the variation of the rate coefficient with temperature at low pressure. The HO2 radicals are produced by a pulse of ultraviolet laser radiation, which dissociates chlorine gas; the chlorine atoms then initiate the chemistry which forms the HO2. The radicals are then detected using tunable diode laser spectroscopy. The technique allows the decay of the radicals to be followed in real time over a time span of tens of milliseconds, from which the rate coefficient can be determined.
There have been 4 previous determinations of the temperature dependence of the rate coefficient at low pressure. Whereas the 3 earlier measurements (Thrush and Tyndall, 1982; Kircher and Sander, 1984; Takacs and Howard, 1986) showed a dependence on temperature, a more recent one (Christensen et al, 2002) suggested that the reaction rate is independent of temperature. However, the new measurements indicate an increase in the rate coefficient at low temperature, in agreement with the earlier studies.
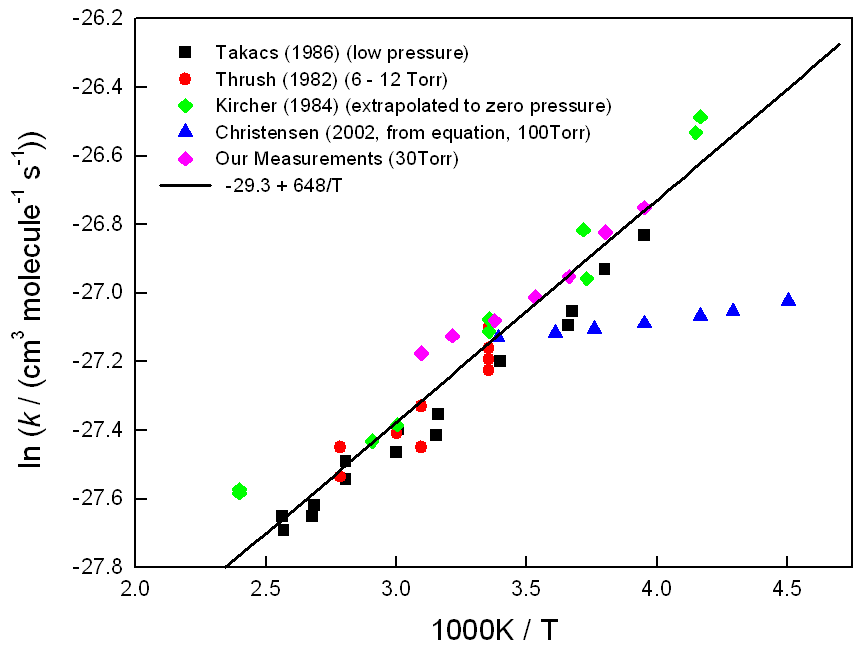 Figure 1. Arrhenius plot showing the variation of the rate coefficient for HO2 + HO2 as a function of inverse temperature at low pressure.
Figure 1. Arrhenius plot showing the variation of the rate coefficient for HO2 + HO2 as a function of inverse temperature at low pressure.
While OH is the major oxidant in the troposphere, it has been suggested that HO2 is able to initiate the oxidation of certain compounds, notably carbonyl compounds such as formaldehyde, acetaldehyde and acetone. A series of experiments are being carried out using both direct laser detection of HO2 and the measurement of stable end products by infrared spectrometry, in order to investigate these reactions. Preliminary results suggest that HO2 reacts reversibly with these carbonyl compounds, but that the overall rate of reaction with acetone is much slower than those of the aldehydes. The reactions may provide a route to the formation of organic acids in the atmosphere.
In a further series of studies, the reactions of HO2 radicals with organic peroxy radicals are been investigated. These reactions were initially thought to form solely organic hydroperoxides, reasonably stable compounds that terminate the oxidative chain reactions. However, in a 2004 study in collaboration with Fresno State University [Hasson et al., 2004] it was shown that OH radicals could be produced in the reaction of HO2 with acetyl peroxy radicals.
HO2 + CH3C(O)O2 → CH3CO2 + OH + O2
The potential for recycling of HO2 to OH in these reactions may be able to explain apparently high levels of OH radicals in relatively clean, forested areas. A systematic study of the OH yields from reaction of HO2 with oxygenated peroxy radicals is currently underway.
References
Christensen, L. E., M. Okumura, S. P. Sander, R. J. Salawitch, G. C. Toon, B. Sen, J.-F. Blavier, and K. W. Jucks, Geophys. Res. Lett. 29 (2002) doi 10.1029/2001GL014525.
Hasson, A. S., G. S. Tyndall, and J. J. Orlando, J. Phys. Chem. A 108 (2004) 5979.
Kircher, C. C., and S. P. Sander, J. Phys. Chem. 88 (1984) 2082.
Takacs, G. A, and Howard, C. J., J. Phys. Chem. 90 (1986) 687.
Thrush, B. A., and G. S. Tyndall, J. Chem. Soc. Faraday Trans. 78 (1982) 1469.
